Breakthroughs
America’s biopharmaceutical companies are transforming patient care.
From harnessing immune systems to fight cancer to delivering gene therapies for rare diseases, our industry is innovating for a healthier America.

Progress for Patient Care
Over the last 25 years, biopharmaceutical innovations have transformed how we fight disease, changing the lives of millions of patients, their families and their communities.



Cancer Care Progress
New cancer medicines contributed to 1.3 million fewer cancer deaths between 2000 and 2016.
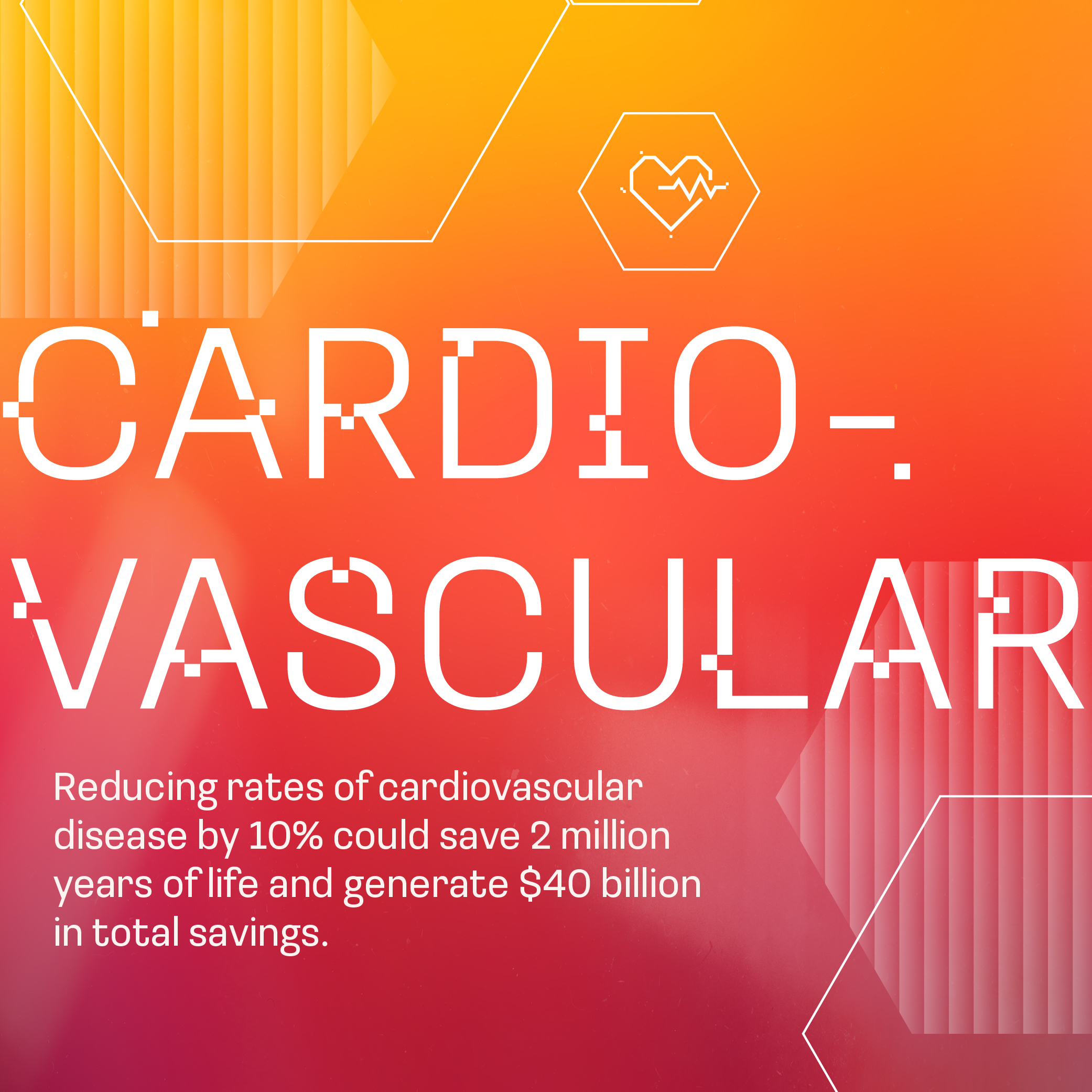
Cardiovascular Outcomes Improved
A new generation of cardiovascular therapies have drastically cut hospitalization and mortality for patients over the last decade.
Mental Health Breakthroughs
New mental health treatments, including the first novel schizophrenia medication in decades, are offering hope to patients.
Chronic Disease Prevention
GLP-1 therapies are transforming care for chronic conditions like diabetes and obesity, while also helping to prevent serious diseases affecting the heart, liver and kidney, as well as sleep apnea and even some cancers.
For over 40 years, the U.S. has led global biopharmaceutical innovation. This success is driven by a robust ecosystem of smart federal policies, public-private partnerships and strong intellectual property protections. These incentives ensure that R&D investments continue to turn scientific discoveries into life-changing treatments for patients in the United States and around the world.
Smart Policy Built the U.S. Innovation Engine
While many promising medicinal ideas once sat undeveloped, biopharma investment now transforms publicly funded research into lifesaving, real-world innovations.
Smart Policy Helped Scale Innovation and Affordability
Limited generic competition, high costs and uneven access has given way to a balanced system that enables innovation while delivering lower costs.
Medicines Are a Vital Tool for Healthier Lives
In the last two and a half decades, medicines have demonstrated their unmatched value to the health system by preventing more costly interventions.
"Thanks to our nation’s incredible biopharmaceutical researchers who work tirelessly to develop treatment options for patients like me, I am able to have a second chance at life. I am blessed to say that now over 25 years later, I am still cancer-free."
Terry
Cancer Survivor


The Promise of Artificial Intelligence in Medicine
Used as a tool, artificial intelligence (AI) in medical research has the potential to identify promising ideas, improve research and development and clinical trials and safely deliver medicines faster.

Breakthroughs are Possible Through Smart Policy
Smart policies that foster innovation are the key to ensuring U.S. biopharmaceutical leadership. Drug price-setting policies and proposals to allow government seizure of patent rights risk undermining the innovation ecosystem that drives breakthroughs.
Sustaining American biopharmaceutical leadership and access to groundbreaking medicines requires a supportive and predictable policy and regulatory environment that encourages collaboration and risk-taking.
Our Work in Action
To maintain America’s global leadership, we need to support the next generation of innovation in medicine.