Innovation Ecosystem

Where’s the Drug Dollar Going?
The path of a prescription medicine from a manufacturer to a patient involves multiple stakeholders and impacts what patients pay at the pharmacy.
In fact, half of every dollar goes to someone who did not make the medicine.
Smart Policies Protect America’s World-Leading Biopharmaceutical Innovation Ecosystem
Policies enacted over the last few decades have shaped an American ecosystem that encourages risk-taking, rewards innovation and turns ideas into medicines.
A healthier future requires not only a commitment to strengthening our world-leading innovation ecosystem but also common sense reforms that put an end to insurer and PBM abuses, fix the IRA, reform the 340B hospital markup program and ensure patient assistance goes to patients.
Generics and biosimilars drive patient and system savings
Our intellectual property system balances innovation and affordability, resulting in a robust pipeline of new medicines and broad access to lower-cost generics and biosimilars. Widespread use of generics and biosimilars keeps costs low; in 2023, the U.S. saved $445 billion by using generics and biosimilars.
Today, 90% of prescriptions in the U.S. are filled with generics, and the average patient copay for a generic medicine is $6.
IP protections fuel competition and patient choice
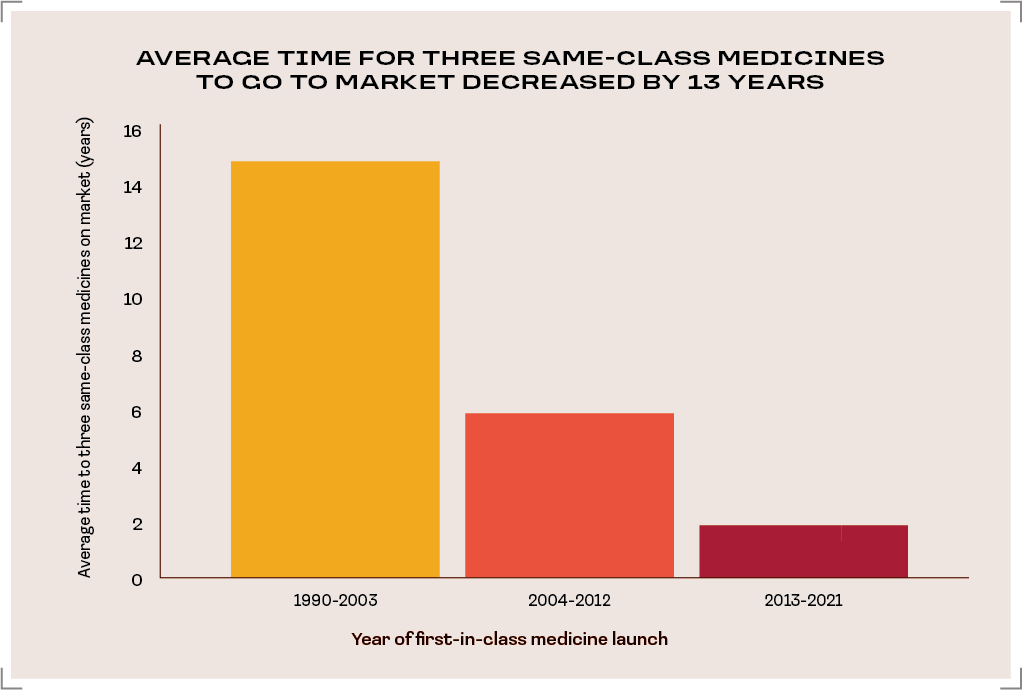
America’s strong intellectual property framework fuels competition and the development of transformative treatments.
The average time until three medicines were available within a class in the United States fell from ~15 years to ~2 years.
Americans have more treatment options than anywhere else in the world
U.S. leadership in global biopharmaceutical innovation has led to Americans having the fastest access to new medicines and more treatment options.

"We need to be innovating and staying abreast of all the potential that science has to offer. That’s why I think that ensuring there are policies that maintain innovation is so critical.”
Sandra Milan
Vice President, Project Team Leadership, Molecular Oncology, Genentech

Innovation Ecosystem Reinforces American Leadership
Our world-leading innovation ecosystem is critical to maintaining our country’s leadership in biopharmaceutical innovation and supporting patient access to new medicines.

Our Work in Action
To maintain America’s global leadership, we need to support the next generation of innovation in medicine.